Anti-GFP tag Mouse mAb
Catalog number :AT0028
The green-fluorescent protein (GFP) functions as a bioluminescence energy transfer acceptor in the jellyfish Aequorea that maximally absorbs light at 395 nm and has an emission spectrum that peaks at 509 nm. GFP has become a very useful tool as a fusion protein that reports gene expression, traces cell lineage and defines subcellular protein localization.
- Overview
- Description
- Mouse monoclonal antibody to GFP
- Reactivity
- green fluorescent protein (GFP), Enhanced green fluorescent protein (EGFP)
- Tested applications
- WB : 1/2000-1/10000.
IHC : 1/200-1/5000.
ICC/IF : 1/200-1/2000.
IP/CoIP : 1/100-1/500.
Not yet tested in other applications. Optimal dilutions/concentrations should be determined by the end user.
- Product Picture
- Specificity
- This antibody detects GFP, EGFP -tagged proteins exogenously expressed in cells or E. coli. This antibody does not detect RFP-tagged proteins.Please note that the GFP, EGFP tags add approximately 27 kDa to the molecular weight of the fusion protein.The antibody recognizes the GFP, EGFP tag, which is fused to either the amino-terminus or carboxy-terminus of the target protein.
- Properties
- Immunogen
- a Green Fluorescent Protein (GFP) recombinant protein.
- Clonality
- Monoclonal, clone number: 7C5
- Isotype
- Mouse IgG2a
- Form
- Liquid, 100 μg at 1mg/mL
Mouse monoclonal antibody in 10 mM phosphate buffered saline (PBS), pH 7.4, 50% glycerol with 1 mg/mL (0.1% w/v) BSA (IgG free, protease free) as a stabilizer and 0.02% (w/v) sodium azide as preservative.
- Storage instruction
- Store at +4°C short term (1-2 weeks). Aliquot and store at -20°C, Avoid freeze / thaw cycle.
- Host
- Mouse
- Applications
- WB Image

Western Blot: GFP Antibody
Lane 1: HEK293T lysate 50µg (transfected pEGFP-N1-POI).
Lane 2: HEK293T lysate 50µg (transfected pEGFP -C1-POI).
Lane 3: Hela lysate 50µg (transfected pEGFP -N1-POI).
Primary antibody: GFP antibody at 1:5000
Observed band size: 48 kDa
Recommended secondary antibody: Goat anti mouse IgG-HRP (Engibody, Catalog number: AT0098)
Recommended ECL: ECL Pico-Detect™ Western Chemiluminescent HRP Substrate (Engibody, Catalog number: IF6747)
- ICC/IF Image
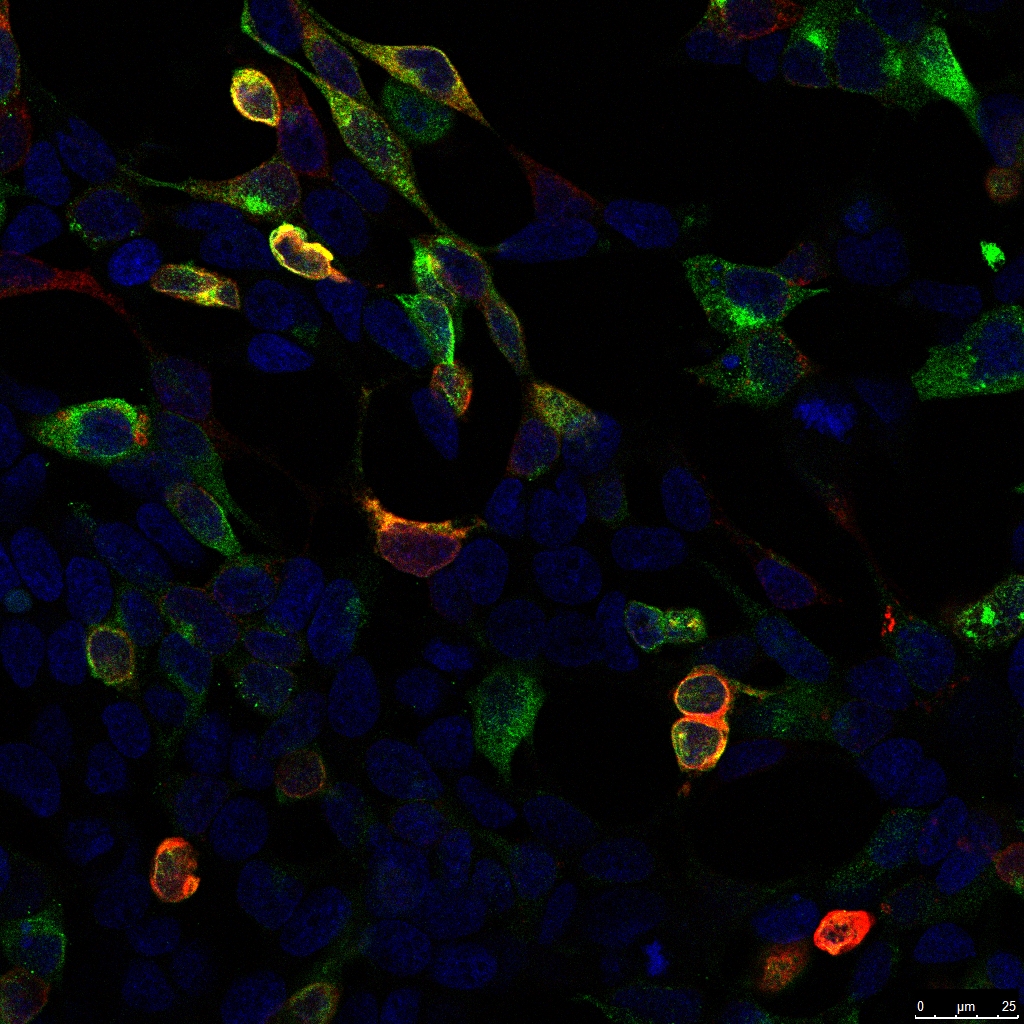
Immunocytochemistry / Immunofluorescence:
GFP Antibody(AT0028) - GFP-transfected 293T cells.mCherry antibody (AT0037) labels mCherry tagged proteinNuclei were counterstained with DAPI.
- IP/CoIP Image
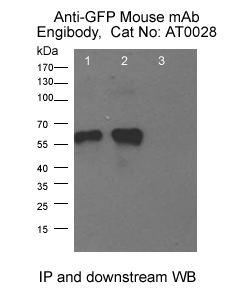
Immunoprecipitation: Anti-GFP tag Mouse mAb (Engibody, Catalog number:AT0028)Analysis of 400 µg cell lysate/extract of GFP-fused gene transfected 293T cellsLane 1: 30 µg lysate/extract of transfected 293T cellLane 2: Immunoprecipitation of GFP-fused protein by 1:500 of GFP tag Mouse mAbLane 3: Control with 1:500 of normal mouse IgGRecommended immunoprecipitation antibody for IP: Anti-GFP tag Mouse mAb (Engibody, Catalog number:AT0028)Recommended protein A/G beads: Protein A/G Agarose Beads (Engibody, Catalog number: IF0001), or Protein A/G Magnetic Beads (Engibody, Catalog number: IF0002)Recommended detection antibody for downstream WB: Anti-GFP tag mouse mAb (Engibody, Catalog number:AT0028)Recommended secondary antibody for downstream WB: MOU-IP Anti-mouse IgG for IP (HRP) (Engibody, Catalog number:AT9204), so that the heavy and light chains from immunoprecipitation antibody can't be seen in WB.
- Protocols
- Western Blotting Procedure
1. Lyse approximately 107 cells in 0.5 mL of ice-cold Cell Lysis Buffer (formulation provided below). This buffer, a modified RIPA buffer, is suitable for recovery of most proteins, including membrane receptors, cytoskeletal-associated proteins, and soluble proteins. This cell lysis buffer formulation is available as a separate product which requires supplementation with protease inhibitors immediately prior to use (Cat. # FNN0011, thermofisher). Other cell lysis buffer formulations, such as Laemmli sample buffer and Triton-X 100 buffer, are also compatible with this procedure. Additional optimization of the cell stimulation protocol and cell lysis procedure may be required for each specific application.
2. Remove the cellular debris by centrifuging the lysates at 14,000 x g for 10 minutes. Alternatively, lysates may be ultra-centrifuged at 100,000 x g for 30 minutes for greater clarification.
3. Carefully decant the clarified cell lysates into clean tubes and determine the protein concentration using a suitable method, such as the Bradford assay. Polypropylene tubes are recommended for storing cell lysates.
4. React an aliquot of the lysate with an equal volume of 2x Laemmli Sample Buffer (125 mM Tris, pH 6.8, 10% glycerol, 10% SDS, 0.006% bromophenol blue, and 130 mM dithiothreitol [DTT]) and boil the mixture for 90 seconds at 100°C.
5. Load 10-30 µg of the cell lysate into the wells of an appropriate single percentage or gradient mini-gel and resolve the proteins by SDS-PAGE.
6. In preparation for the Western transfer, cut a piece of PVDF slightly larger than the gel. Soak the membrane in methanol for 1 minute, then rinse with ddH2O for 5 minutes. Alternatively, nitrocellulose may be used.
7. Soak the membrane, 2 pieces of Whatman paper, and Western apparatus sponges in transfer buffer (formulation provided below) for 2 minutes.
8. Assemble the gel and membrane into the sandwich apparatus.
9. Transfer the proteins at 140 mA for 6°C 0-90 minutes at room temperature.
10. Following the transfer, rinse the membrane with Tris buffered saline for 2 minutes.
11. Block the membrane with blocking buffer (formulation provided below) overnight at 4°C or for one hour at room temperature.
12. Incubate the blocked blot with primary antibody at a 1:1000 starting dilution in Tris buffered saline supplemented with 1% Ig-free BSA and 0.1% Tween-20 overnight at 4°C or for two hours at room temperature.
13. Wash the blot with several changes of Tris buffered saline supplemented with 0.1% Tween 20.
14. Detect the primary antibody band using an appropriate secondary antibody, such as goat anti-rabbit IgG conjugated HRP (Cat. # AT0097-100ul, Engibody) or goat anti-mouse IgG conjugated HRP (Cat. # AT0098-100ul, Engibody) in conjunction with your chemiluminescence reagents and instrumentation.
- Troubleshooting Guide
- Western Blot Troubleshooting
1. No signal
2. High background
3. Non specific bands
4. Incorrect band size
5. White bands on black/grey blot
6. ‘Smear’
1. Troubleshooting: NO SIGNAL
Sample preparation
• Untested species? Positive control. Check alignment.
• Not enough antigen in the sample or loaded onto the gel.
Transfer to the membrane
• Poor transfer to membrane.
• Excessive washing.
Blocking
• Too much blocking. Optimize blocking agent, concentration and time
• Cross-reaction blocking agent / antibody.
Antibody incubation and detection
• Not enough antibody.
• Incorrect secondary antibody.
• Detection kit is old - substrate inactive.
2. Troubleshooting: HIGH BACKGROUND
Transfer to the membrane
• Choice of membrane.
Blocking
• Need to block for longer.
• Optimize blocking agent, concentration.
• Cross-reaction between blocking agent and primary or secondary.
• Phospho-specific proteins: use Casein to block.
Antibody incubation and detection
• Primary antibody concentration too high.
• Incubation temperature too high.
3. Troubleshooting: NON SPECIFIC BANDS
Sample preparation
• Cell lines can accumulate differences in their protein expression profiles.
• Protein has multiple modified forms, altering mobility.
• Target protein is in fragments – degradation by proteases or cleaved.
• Splice variants / isoforms or possibly proteins from the same family.
• Multimers (dimers) of protein (reduce and denature).
• Bands may be non-specific. Run a no primary control
Blocking
• Insufficient blocking. Optimizing concentration, time.
Antibody incubation and detection
• Primary or secondary antibody concentration too high.
• Antibody has not been purified.
4. Troubleshooting: INCORRECT BAND SIZE
Sample preparation
• Ensure sample is reduced and denatured unless stated otherwise on the datasheet.
• Check for isoforms.
• Is the sample a recombinant fragment? These will run at a different size.
5. Troubleshooting: WHITE BANDS/ BLACK BLOT
• Too much primary and/or too much secondary antibody.
6. Troubleshooting: ‘Smear’
• Overloading, Protein degradation.
Related Products
| Catalog number | AT0055 |
| Catalog number | AT0063 |
| Catalog number | AT0064 |
| Catalog number | AT0065 |
| Catalog number | AT0083 |
| Catalog number | AT0089 |
| Catalog number | AT0022 |
| Catalog number | AT0023 |
| Catalog number | AT0024 |
| Catalog number | AT0025 |
| Catalog number | AT0026 |
| Catalog number | AT0027 |
| Catalog number | AT0029 |
| Catalog number | AT0030 |
Reviews
loading...