Anti-Myc tag Mouse mAb conjugated Agarose Beads
Catalog number :AT0080
Anti-Myc Agarose beads (Affinity gel) is a mouse monoclonal antibody that is covalently attached to agarose beads by hydrazide linkage. The antibody binds Myc tag at the N-terminal, C-terminal and internal locations of fusion proteins.
- Overview
- Description
- Anti-Myc tag mouse monoclonal antibody conjugated Agarose Beads
- Reactivity
- Species independent
- Tested applications
- Immunoprecipitation (IP), Co-Immunoprecipitation (Co-IP), Myc tagged fusion protein purification
In IP, 40 µL of beads slurry (20 µL of settled beads) for ~500-1000 µL of cell lysate.Optimal dilutions/concentrations should be determined by the end user.
- Product Picture

- Specificity
- The antibody detects and captures Myc-Tagged fusion proteins overexpressed in cells or E. coli. Myc-tag sequence motif EQKLISEEDL can be recognized at the N-terminus, C-terminus, or internal site of the fusion protein.
Endogenous nuclear protein c-myc (P62) is NOT bound.
- Properties
- Immunogen
- This monoclonal antibody is produced by immunizing mice with a synthetic peptide (KLH-coupled) containing EQKLISEEDL.
- Clonality
- Monoclonal antibody, clone number: 7G10
- Isotype
- Mouse IgG1
- Form
- The product is supplied as a 50% suspension in 10 mM phosphate buffered saline (PBS), pH 7.4, 0.02% (w/v) sodium azide as preservative.
- Storage instruction
- Stored at 4 °C. Do not freeze
- Structure Image
- Beads Diameter
- ~ 90 µm (cross-linked 8% agarose beads)
- Binding Capacity
- >1 mg of Myc-tagged fusion protein per 1 mL of beads slurry.
- Applications
- Application Figure 1
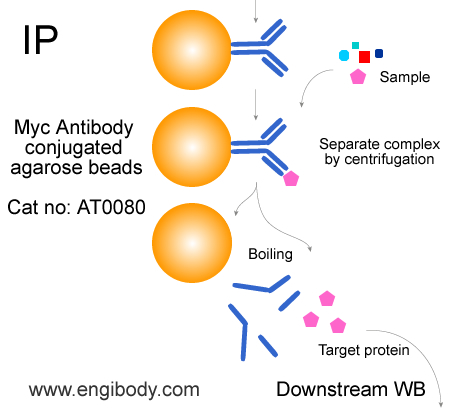
- Application Figure 2
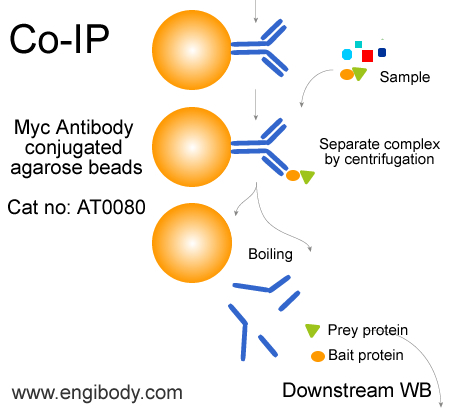
- Application Figure 3
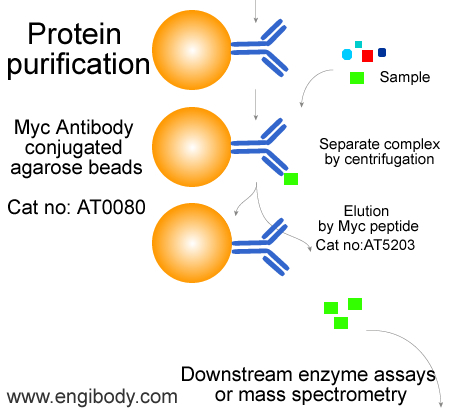
- Protocols
- Attention: Agarose Beads should be resuspended well before used in IP and purification.
Protocol for Immunoprecipitation (IP/CoIP) of Myc tagged Fusion Proteins from mammalian cell extract (For soluble cytoplasmic protein)
Solutions and Reagents1X Cell Lysis Buffer: 50 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100. Recommend adding 1 mM PMSF before use.1X Wash Buffer: TBS (50 mM Tris HCl, 150 mM NaCl, pH 7.5)ProcedureAttention:1. If your target protein is soluble nuclear protein in nucleus (Excluding nuclear membrane integrated protein and DNA-binding protein), please use NE-PER Nuclear and Cytoplasmic Extraction kit (Thermo, Cat no:78833) to extract nuclear protein. Or please use RIPA lysis buffer to lyse the cell, and add PIC, 1mM PMSF, 2.5mM Mgcl2 and 1mg/ml DNase I.2. It is required to reserve 50 µl of protein extract for each sample as the INPUT control, which is directly used in the downstream WB experiment.Sample Lysate PreparationRecommended procedure for mammalian cellsFor a 70–90% confluent 100 mm dish (106–107 cells), use 1 ml of IP lysis buffer (50 mM Tris HCl, pH 7.4, with 150 mM NaCl, 1 mM EDTA, and 1% TRITON X-100). If the expression level of the Myc fusion protein is relatively low, lyse the cells with a reduced volume of IP lysis buffer. It is highly recommended to add a protease inhibitor cocktail (sigma, Catalog Number P8340) to the IP lysis buffer (10 ml per 1 ml of lysis buffer), especially if the lysate is to be stored for further use.
1. Wash adherent or suspension cells as appropriate:
Adherent Cells - Remove the growth medium from the cells to be analyzed. Rinse the cells twice with PBS buffer, being careful not to dislodge any of the cells. Discard the PBS. Add lysis buffer (106–107cells/ml).
Cells in Suspension - Collect the cells into an appropriate conical centrifuge tube. Centrifuge for 5 minutes at 420×g. Decant the supernatant and discard. Wash the cells twice by resuspending the cell pellet with PBS and centrifuge for 5 minutes at 420×g. Decant the supernatant and discard. Resuspend the cell pellet in lysis buffer (106–107 cells/ml).
2. Incubate the cells for 15–30 minutes on a shaker.
3. For adherent cells only, scrape and collect the cells. For cells in suspension, proceed to step 4.
4. Centrifuge the cell lysate for 10 minutes at 12,000×g.
5. Transfer the supernatant to a chilled test tube. For immediate use, keep on ice. If the
supernatant is not to be used immediately, store it at –70 °C.
2. Pre washing the gel beads to equilibrate beads and remove the product buffer.
1. Thoroughly resuspend the anti- myc Agarose gel by inverting the product tube or by pipette tip. Note: this step is very important. Make sure to resuspend the gel very well. Do not Vortex the agarose beads.
2. Immediately transfer 40ml of the gel suspension to a fresh tube, add 0.5ml TBS (50 mM Tris HCl, with 150 mM NaCl, pH 7.4), then resuspend beads and mix TBS very well. centrifuge the gel suspension at 5,000×g for 30 seconds, remove the supernatant carefully, make sure that most of the wash buffer is removed and no gel beads is discarded. This step should be repeated for 3-4 times.
In case of numerous immunoprecipitation samples, wash the gel beads needed for all samples together. After washing, divide the gel according to the number of samples tested. Each wash should be performed with TBS at a volume equal to 20 times the total packed beads volume.
Note: For the gel transfer, use a clean, plastic pipette tip with the end enlarged to allow the gel to be transferred easyly.
3. Myc Fusion Protein Immunoprecipitation
1. Add 200–1,000 ml of E. coli extracts or cell lysates to the washed gel. If necessary, bring the final volume to 1 ml by adding IP lysis buffer (50 mM Tris HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% TRITON X-100).
Note: The volume of E. coli extracts or cell lysates to be used depends on the expression level of Myc fusion protein in the transfected cells or bacterial. The amount of Myc fusion protein to be precipitated depends on the detection method. 200 ng of protein is sufficient for an activity assay or for an immunoblot analysis. For SDS-PAGE analysis with Coomassie blue or silver staining, use 1 mg of Myc fusion protein.
For the negative control, add only 1 ml of IP lysis buffer with no protein.
2. Agitate or shake all samples and controls gently (a roller shaker is recommended) for 2 hours. In order to increase the binding efficiency, the binding step may be extended overnight.
4. Washing immune complexes to remove nonspecific binding proteins.
1. Centrifuge the resin for 30 seconds at 5,000–8,000×g. Remove the supernatants with a narrow-end pipette tip.
2. Wash the gel three times with 0.5 ml of TBS (50 mM Tris HCl, with 150 mM NaCl, pH 7.4). Make sure all the supernatant is removed by using a Hamilton syringe or equivalent device.
5. Elution and obtaining of the Myc -fusion protein
Three elution and obtaining methods are recommended according to protein characteristics or further usage:
Option A: Protein elution under native conditions by competition with Myc peptide. The elution efficiency is very high using this method.
Option B: Elution under acidic conditions with 0.1 M glycine HCl, pH 3.5. This is a fast and efficient elution method. Equilibration of the eluted proteins with neutralizing buffer (0.5 M Tris HCl, with 1.5 M NaCl, pH 7.4) may help preserve its activity.
Option C: Boil with sample loading buffer, Centrifuge and get the supernatants for gel electrophoresis and western blotting.
Option A: Elution with Myc peptide
1. Prepare Myc peptide solution. Dissolve Myc peptide (Engibody, Catalog No. P5203) in 0.5 M Tris HCl, pH 7.5, with 1 M NaCl at a concentration of 25 mg/ml. Dilute 5-fold with water to prepare a Myc peptide stock solution containing 5 mg/ml of Myc peptide. For elution, add 3 ml of 5 mg/ml of Myc peptide stock solution to 100 ml of TBS (150 ng/ml final concentration).
2. Add 100 ml of Myc peptide elution solution to each sample and control resin.
3. Incubate the samples and controls with gentle shaking for 30 minutes at 2–8 °C.
4. Centrifuge the gel for 60 seconds at 8,000×g. Transfer the supernatants to fresh test tubes using a Hamilton syringe or equivalent device. Be careful not to transfer any gel beads.
5. For immediate use, for example, western blotting, store the eluted proteins at 2–8 °C. Store at –20 °C for long term storage.
For downstream western blotting analysis, add appropriate SDS-PAGE Sample loading Buffer to the eluted proteins and boil, then the samples and controls are ready for loading on SDS-PAGE and immunoblotting using anti-Myc or specific antibodies against the fusion protein.
Option B: Elution with 0.1 M glycine HCl, pH 3.5
The procedure should be performed at room temperature.
Note: Do not leave the gel beads in this buffer more than 20 minutes.
1. Add 100 ml of 0.1 M glycine HCl buffer (pH 3.5) to each sample and control gel.
2. Incubate the samples and controls with gentle shaking for 5 minutes at room temperature.
3. Centrifuge the gel for 60 seconds at 8,000×g.
4. Transfer the supernatants to fresh test tubes using a Hamilton syringe or equivalent device. Be careful not to transfer any gel beads.
5. In order to equilibrate the eluant, immediately add 10 ml of neutralizing buffer (0.5 M Tris HCl, with 1.5 M NaCl, pH 7.4) to each sample.
6. For immediate use, for example, western blotting, store the eluted proteins at 2–8 °C. Store at –20 °C for long term storage.
For downstream western blotting analysis, add appropriate SDS-PAGE Sample loading Buffer to the eluted proteins and boil, then the samples and controls are ready for loading on SDS-PAGE and immunoblotting using anti-Myc or specific antibodies against the fusion protein.
Option C: Boil with sample loading buffer, Centrifuge and get the supernatants for gel electrophoresis and western blotting.
The procedure should be performed at room temperature. Sample loading buffer should be at room temperature before use. In order to minimize the denaturation and elution of the antibody, no reducing agent (2-mercaptoethanol or DTT) should be included in the Sample loading buffer. The addition of reducing agents will result in the dissociation of the heavy and light chains of the immobilized anti Myc antibody (25 and 50 kDa bands). If reducing conditions are absolutely necessary, a reducing agent may be added. The final concentration of 2-mercaptoethanol or DTT in the 1×sample loading buffer (62.5 mM Tris HCl, pH 6.8, with 2% SDS, 10% (v/v) glycerol, and 0.002% bromphenol blue) should be 5% or 50 mM, respectively.
Note: SDS in the sample buffer will denature the anti Myc antibody, and the anti Myc affinity gel cannot be reused after treatment with the SDS-PAGE Sample loading buffer.
1. Add 20 mL of 2×sample loading buffer (125 mM Tris HCl, pH 6.8, with 4% SDS, 20% (v/v) glycerol, and 0.004% bromphenol blue) to each sample and control.
2. Boil the samples and controls for 3 minutes.
3. Centrifuge the samples and controls at 8,000×g for 60 seconds to pellet any undissolved agarose beads. Transfer the supernatants to fresh test tubes with a Hamilton syringe or a narrow-end Pasteur pipette. The samples and controls are ready for loading on SDS-PAGE and immunoblotting using anti-Myc or specific antibodies against the fusion protein.
Related Products
| Catalog number | AT0023 |
| Catalog number | AT0078 |
| Catalog number | AT0540 |
| Catalog number | AT0079 |
| Catalog number | AT0541 |
| Catalog number | AT0081 |
| Catalog number | AT0082 |
| Catalog number | AT0083 |
| Catalog number | AT0084 |
| Catalog number | AT0085 |
| Catalog number | AT0086 |
| Catalog number | AT0087 |
| Catalog number | AT0088 |
Reviews
loading...