Purification Protocol
Purification of soluble GST-tagged proteins under native conditions.
(Attention: Denatured protein cannot be purified)
1. Prepare an iron stand for clamping the purification column.
Fix the Gravity Column on the iron frame, put the lower frit into the empty column.
2. Sample Preparation
2.1. For protein expressed in E. coli or yeast cytoplasm.
1) Harvest cells from a 50 mL culture by centrifugation at 4°C (e.g., 5,000 rpm for 15 minutes).
2) Resuspend the cells in 10 mL of Equilibration buffer (LE buffer) with appropriate amount of PMSF (Final working concentration can be 1mM) and add lysozyme (Final working concentration is 0.2-0.4 mg/mL), or other protease inhibitors cocktail added.
3) Sonicate the solution on ice using 180 one-second bursts at high intensity with a three-second cooling period until the bacterial liquid is clear.
Optional: If the lysate is too viscous, add RNase A (10 µg/mL) and DNase I (5 µg/mL) and incubate on ice for 10-15 minutes.
4) Centrifuge the lysate at 12,000 rpm for 15-25 minutes at 4°C to pellet the cellular debris. Take the supernatant and store it in a new centrifuge tube. Apply the supernatant onto the GSH column in next steps.
2.2. For proteins secreted into culture medium by yeast, insect, or mammalian expression systems.
1) If the culture supernatant does not contain SDS, urea, or any other denaturing agents that might affect the binding of GST tagged protein and GSH column, it can be applied directly to the column. Otherwise, perform the following procedures.
2) Dialyze the sample against 1 × PBS before applying it onto the column.
3) For large volume of supernatant, concentrate the proteins by ammonium sulphate precipitation, dialyze the dissolved protein solution against 1 × PBS, and then apply the solution onto the GSH column.
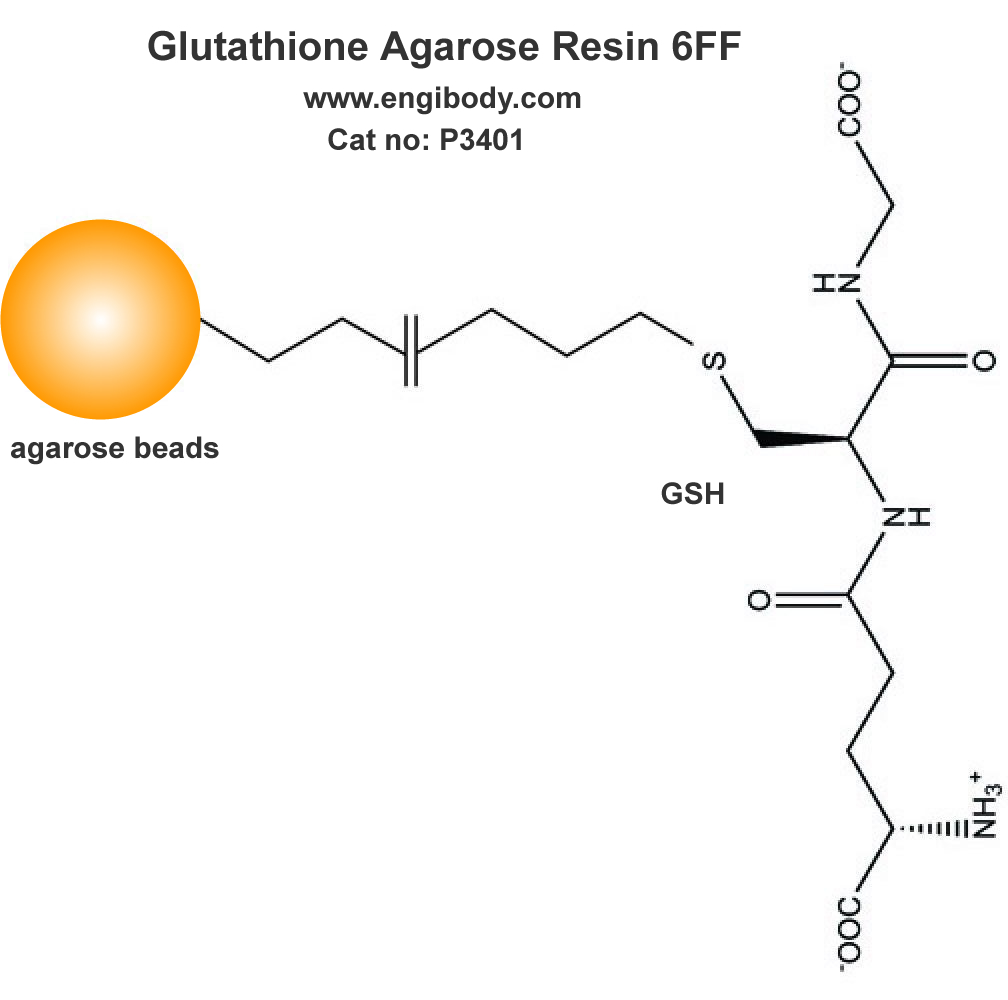
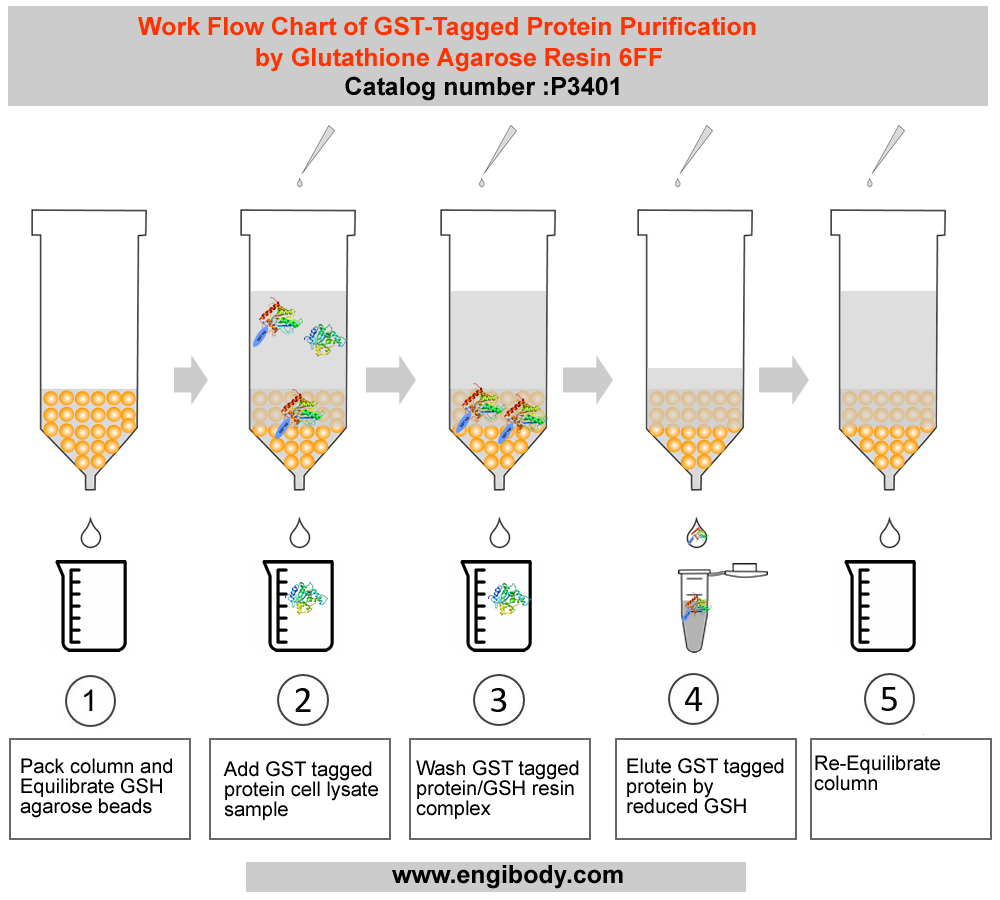
3. Pack column and Equilibrate Glutathione (GSH) Resin.
1). Mix the slurry by gently inverting the bottle several times to completely suspend the resin.
2). Transfer an appropriate volume (2 - 4mL) of the slurry to the column. Allow the resin to settle down and the storage buffer to drain from the column.
3). Equilibrate the column with 5 × bed volumes of Equilibration buffer (LE buffer) until A280 is stable.
4. Native GST-tagged Proteins Purification
1). Add the clear sample containing target GST-tagged protein onto the equilibrated gravity column, keep the sample in column for at least 2 min, ensure that the sample fully binds with the resin, and collect the flowthrough with a flow-rate of 0.5 - 1 mL per minute, then add the flowthrough onto the column again, repeat the previous operation, this can increase the binding efficiency.
2). Wash the column with 10 - 15 × bed volumes of Wash buffer until A280 is stable at the flow-rate of 1 mL per minute. Remove non-specific adsorbed proteins. Collect the wash fractions.
3). Use 5-10 × bed volume of Elution buffer (added GSH) to elute, collect in fractions, collect one tube for each bed volume, and test separately, which can not only ensure that all binding target proteins are eluted, but also obtain high-purity and high-concentration proteins.
4). Dialyze it against 20 mM Tris-HCl (pH 8.0) or 1 × PBS (pH 7.4) according to the specific application of the target protein.
5. Re-Equilibrate Glutathione (GSH) Resin (which is in gravity column).
3 × bed volumes of Equilibration Buffer (LE buffer) and 5 × bed volumes of deionized water are used to equilibrate the resin in the order. Store the resin in 3 × bed volumes of 1XPBS (20% ethanol) at 4°C-30°C to prevent bacterial pollution of the resin.
6. SDS-PAGE Test
Use the fractions from purification process (including flow through, wash fractions and elution fractions) and whole cell lysate as samples in SDS-PAGE test, so that we can evaluate the purification effect.
After using the resin for several times, if the binding capacity drops very much or the color of the resin has changed significantly, then Cleaning of the GSH resin should be performed.
Resin Cleaning (Optional Step)
GSH resin can be reused several times without regeneration, but with the increase of non-specifically bound proteins and protein aggregation, the flow rate and binding capacity are reduced, and the resin needs to be washed.
To remove some precipitated or denatured substances, the following method is recommended:
Wash with 2 column bed volumes of 6M guanidine hydrochloride solution, followed immediately by 5 column bed volumes of PBS, pH 7.4.
To Eliminate non-specific adsorbents caused by hydrophobic adsorption:
Wash with 3-4 column bed volumes of 70% ethanol or 2 column bed volumes of 1% Triton X-100, followed immediately by 5 column bed volumes of PBS, pH 7.4.